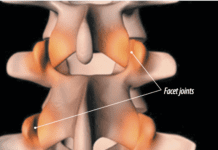
A new low-dose formulation of the oral capsule diclofenac (Zorvolex) has been approved by the Food and Drug Administration (FDA) for the treatment of osteoarthritis (OA) pain (Aug. 25, 2014). The formulation consists of submicron particles that are 20 times smaller than in the conventional drug, which provides a greater surface area coverage and more rapid drug dissolution and absorption, according to the drugs manufacturer, Iroko Pharmaceuticals. A phase III trial reviewed the efficacy and safety of diclofenac in 305 adults age 40 and older with radiologically evident OA of the hip or knee who routinely used nonsteroidal anti-inflammatory drugs (NSAIDs) or acetaminophen (Tylenol). Taking 35 milligrams of diclofenac either twice or three times daily led to decreases in pain for both groups, researchers reported in Current Medical Research and Opinion (Aug. 2014).
To continue reading this article or issue you must be a paid subscriber.
Sign in