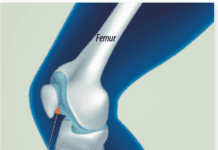
The U.S. Food and Drug Administration (FDA) has approved a cementless ankle replacement system for arthritic ankles that is designed to preserve range of motion in the joint. The new prosthesis, called the Scandinavian Total Ankle Replacement (STAR) System and produced by Small Bone Innovations Inc. (SBI), is a mobile-bearing, three-component design consisting of individual tibial (shin bone) and talar (ankle bone) implants separated by a mobile polyethylene spacer. It is claimed to allow more movement in the joint and result in less wear and loosening than other devices. It is designed as an alternative to fusion surgery, which involves cementing the tibia to the talus. Fusion stabilizes the ankle, but decreases the ability to move the foot up and down.
To continue reading this article or issue you must be a paid subscriber.
Sign in