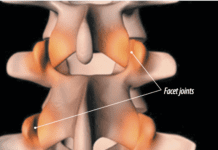
If you have chronic back pain, you may be tempted to try one of todays most widely touted techniques-non-surgical spinal decompression. In essence, this is a fancy term for motorized traction devices or so-called therapeutic tables. A review article published a few years ago in the journal Chiropractic Osteopathy looked at research available on the tables-a few small studies-and concluded that use of the technique is rarely warranted, especially when, according to the author of the study, many scientifically sound, less expensive alternatives are available.
To continue reading this article or issue you must be a paid subscriber.
Sign in